How Does a Nuclear Bomb Work? 💣
Morning nuke!

I’ve been all caught up in this Barbenheimer summer we’ve been having.

And this Oppenheimer guy? He created the most destructive weapon on the planet.
So I spent the entire movie thinking about how I was going to tell you how a nuclear bomb works.
But before we do this, just remember: with great power comes great responsibility. I don’t want to see any nuclear bombs attributed to a Smart Nonsense email.
That would be silly.
So let’s take it back. 1898.
Marie Curie and her husband are in their lab.

Ya, they’re just fingering Uranium like it’s no big deal.
Because, well, it wasn’t a big deal until they noticed something peculiar:

Their Uranium had some mysterious energy that randomly traveled through paper or metal and could expose a photographic plate.
They called this energy “radioactivity” – unstable atoms that decayed at random, unpredictable times.
But how was this radioactivity producing energy that developed a photographic plate?

So?


Oh, that’s what that famous equation does. Cool. Thanks Einstein.
Unfortunately all that fingering of radioactive material catches up to Marie Curie.


She died.
So these two guys – Walton & Cockcroft – take over to see if they can control radioactive decay.
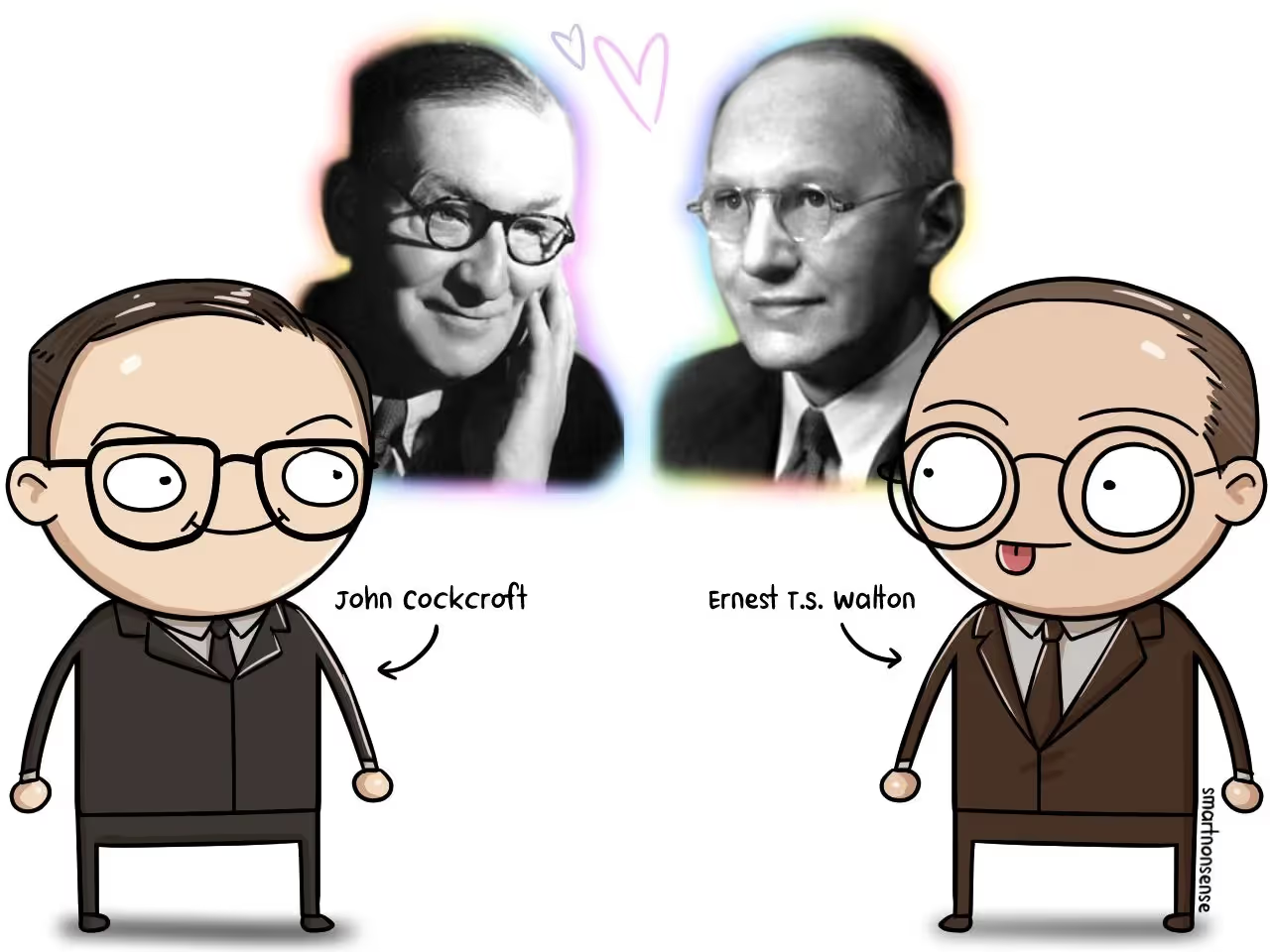
If they could, they’d unlock the energy Einstein was talking about.
So they get a GIANT electric field.

And start blasting protons at lithium nuclei.

The lithium decays! It works!

Just one subatomic problem..
Even with their giant electric field, it doesn’t work that well.
Because protons are positively charged.

And so is the nucleus of an atom.

Positive to positive means they want to repel each other.

It takes an ENORMOUS amount of energy to overcome that repulsion.
This method for unleashing the power of radioactive decay could never work.
It required too much energy.
But in 1932 something amazing happens:

See that fellow particle enthusiast, James Chadwick?
He just discovered the neutron right in front of you.
How?
Well look closely at his magnet.

Those particles flying through his apparatus unimpeded by the magnetic field are neutrons – a neutral subatomic particle.
A particle with no charge.
So unlike our positive atomic nucleus repelling positive protons, neutrons could slam into a nucleus.
For free.

Okay, nuclear bomb time.
Put your goggles on.

Now with our neutral neutrons, we can bombard an unstable atom like Uranium-235.

And when you do that, 2 more neutrons pop off in the reaction

Those 2 neutrons require no extra energy to hit another nucleus.

2 more neutrons pop off!

This 2-neutron-thing goes exponential.
You have a runaway chain reaction on your hands:

And in 1kg of Uranium-235, there’s a trillion TRILLION atoms for this nuclear dance to occur.
Each of those mini E=mc2 mass changes add up to a LOT of released energy when we’re talking about trillion trillions…
We’ve created a nuclear bomb.

This sheer power is what made Einstein and Oppenheimer so butt hurt about inventing the bomb in the first place.
The neutron genie was officially out of the bottle.
Stay Cute,
Henry & Dylan 🌈
If you’re that sexy friend, subscribe here.

Get smart about nonsense🌈
Join 100,000+ subscribers and get our daily comic explaining nerdy stuff like you’re 5.